MEETING THE NEEDS OF OCULAR SURFACE DISEASES
PEDs can result in significant complications, including infection and vision loss.¹
Disruption of the tear film is a hallmark of dry eye disease (DED), which can ultimately give rise to sight-threatening epithelial defects and corneal ulcers. Therefore, a stable preocular tear film is integral in maintaining a healthy ocular surface.²
Corneal epithelial defects are some of the most common ocular pathologies that present to eye care professionals. Persistent corneal epithelial defects (PEDs) result from the failure of rapid re-epithelialization and closure within 10-14 days after a corneal injury, even with standard supportive treatment.³⁻⁴ Corneal epithelial defects are focal areas of epithelial loss most frequently caused by mechanical trauma, corneal dryness, neurotrophic keratitis, post surgical changes, or infection.⁵ If left untreated, PEDs can result in significant complications, including infection and vision loss.¹
Amniotic membrane acts as a physical barrier, protecting the injured corneal surface from eyelid friction and by preventing water loss through desiccation.
Furthermore, Thia et al demonstrated that when used as a graft to treat chronic corneal pathologies such as PEDs and limbal stem cell deficiencies, amniotic membrane serves as a substrate for the migration, adhesion and proliferation of corneal epithelial cells and limbal stem cells.⁵
Amniotic membrane graft applications as a cover or barrier may include, but are not limited to, corneal and conjunctival related injuries or defects such as corneal epithelial defects, pterygium repair, fornix reconstruction and other procedures.
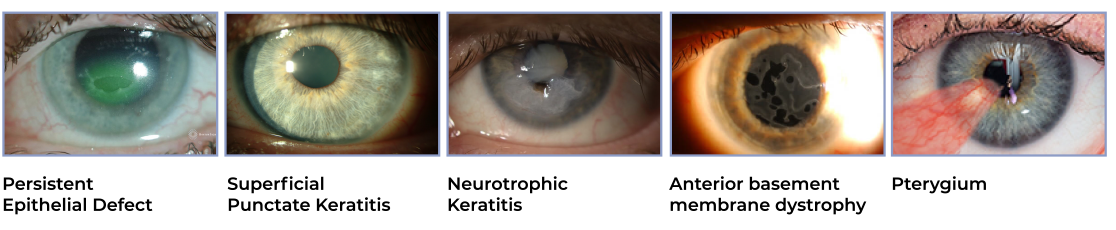
Common diagnoses resulting in or associated with corneal defects include:
Click to Enlarge Image

-Josh Johnston, OD, FAAO
BIOVANCE® 3L OCULAR
Indications For Use
BIOVANCE 3L Ocular is an allograft intended for use as a biological membrane covering that provides an extracellular matrix. As a barrier membrane, BIOVANCE 3L Ocular is intended to protect the underlying tissue and preserve tissue plane boundaries. Applications include, but are not limited to, corneal and conjunctival related injuries or defects such as corneal epithelial defects, pterygium repair, fornix reconstruction, and other procedures.
BIOVANCE 3L Ocular is a pure human amniotic tissue with an intact basement membrane¹:
- Devoid of cells, DNA, and cellular debris
- Retention of natural proteins (e.g. collagen, laminin, fibronectin)
- Serves as a cell-friendly scaffold for cell attachment and migration²
- Cell attachment is a natural stimulus for the orderly release of growth factors and cytokines¹
To learn more visit our Resources page
Designed for Premium Handleability
Biovance® 3L Ocular is a three-layer decellularized, dehydrated, human amniotic membrane.
Cut and assembled as a unique laminated tri-layer design with the stromal side of amniotic membrane on both sides of the scaffold facing out to ensure the correct side interfaces with the ocular surface regardless of the orientation of the scaffold.
Biovance® 3L Ocular’s three layer design enhances its handling properties, without the need for a ring.
Benchtop Study Findings
Cell attachment is a natural stimulus for the orderly release of growth factors and cytokines¹.
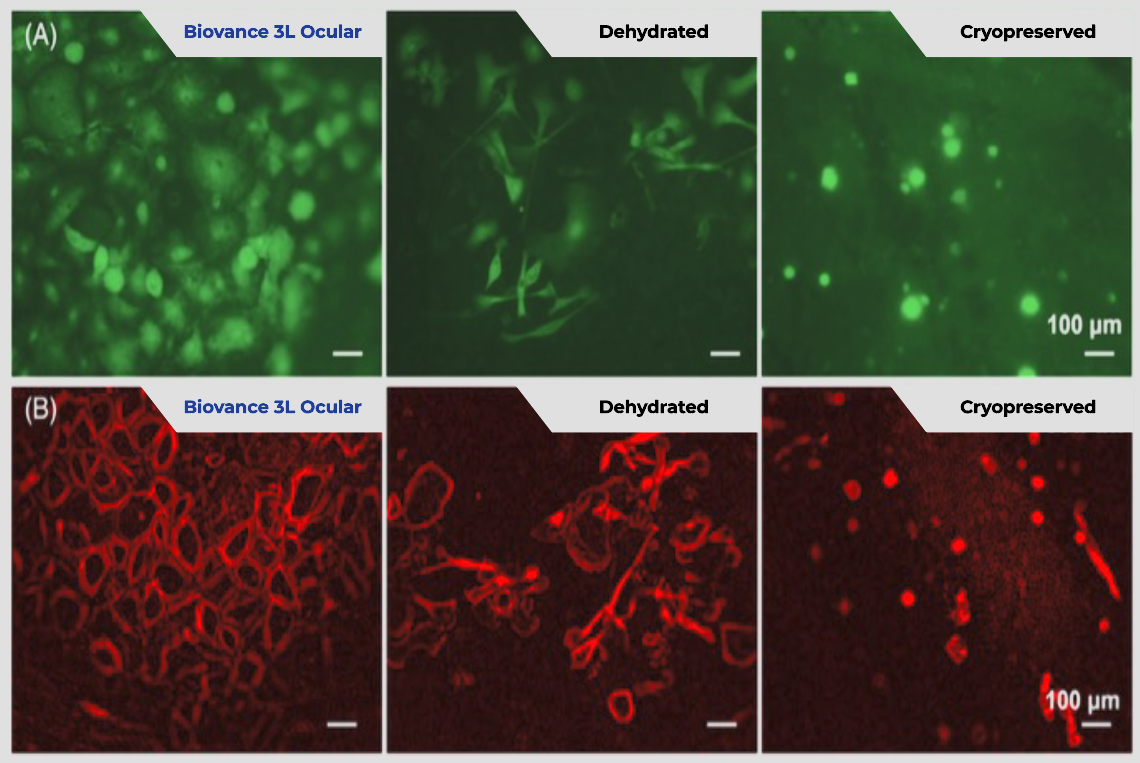
With its unique 3-layer amnion basement membrane construction, BIOVANCE 3L Ocular is designed for superior handling and creating an optimal matrix for cell viability, adhesion, and proliferation, providing a cell-friendly environment as demonstrated in a comparative benchtop study²’³:
- Ocular epithelial cell viability significantly greater than ChAM and DhAM (p<0.001)²
- Ocular epithelial cell adhesion significantly greater as compared to ChAM and DhAM (p≤0.011)²
- Ocular epithelial cell proliferation rate significantly greater than ChAM (p<0.001)²
* ChAM=cryopreserved human amniotic membrane; DhAM=dehydrated human amniotic membrane
Click to Enlarge Image
To learn more visit our Resources page
Ocular epithelial cell viability with Biovance 3L Ocular is significantly greater than ChAM or DhAM at day 4.
Human corneal epitheal cells were seeded on the different scaffolds, cultured, and stained with Calcein AM to visualize viable cells at Day 4 (A). The morphology of human corneal epithelial cells on scaffolds was monitored by actin staining on Day 4 (B).
Click to Enlarge Image
Top 10 reasons to use Biovance 3L Ocular
- Unique 3-layer design
- Easy to handle
- No upfront preparation
- Decellularized (no donor pro-inflammatory cell debris)
- Protects underlying tissue
- Ringless design
- Bidirectional (can be applied on either side)
- Room temperature storage
- 10-year shelf life
- Access support for patients (e.g. benefits verification)
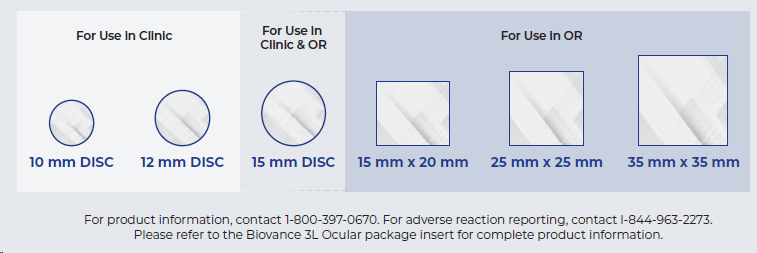
BIOVANCE 3L Ocular is available in 6 convenient shapes & sizes
Click to Enlarge Image
Biovance 3L Ocular Clinical Application Process Video
Order Biovance 3L Ocular Now
Using our Order Form
Biovance 3L Ocular is contraindicated in patients with a known hyper-sensitivity to BIOVANCE 3L Ocular. If a patient has an adverse reaction related to the use of BIOVANCE 3L Ocular, immediately discontinue its use. BIOVANCE 3L Ocular should not be used on clinically infected wounds. The pouch contents are sterile if the pouch is unopened and undamaged. Do not use if package seal is broken. Discard material if mishandling has caused possible damage or contamination. Do not resterilize. BIOVANCE 3L Ocular must be used prior to the expiration date on the product pouch. BIOVANCE 3L Ocular should not be used together with a collage-nase product on the wound.
For product information, contact 1-800-397-0670.
For adverse reaction reporting, contact 1-844-963-2273.
Please refer to the Biovance 3L Ocular package insert for complete product information.
1.Vaidyanathan U. et al; Med Hypothesis Discov Innov Ophthalmol. 2019 Autumn; 8(3): 163–176
2.Mead et al; Taiwan J Ophthalmol 2020;10: 13-21
3.www.ncbi.nlm.nih.gov%2Fpmc%2Farticles%2FPMC6778469%2F%23B4
4.www.ncbi.nlm.nih.gov%2Fpmc%2Farticles%2FPMC6778469%2F%23B5
5.Thia et al; Surv Ophthalmol. 2023 Nov-Dec;68(6):1093-1114. doi: 10.1016/j.survophthal.2023.06.001. Epub 2023 Jun 8.
6.Biovance 3L Ocular Package Insert.
7.Ambrosio R. J Refract Surg 2008; 24:396-407.
8.Fournie PR, Gordon GM, Dawson DG, et al. Arch Ophthalmol 2010; 128:426-436.
9.Venkateswaran N, Luna RD, Gupta PK. Ocular surface optimization before cataract surgery. Saudi J Ophthalmol. 2022 Aug 29;36(2):142-148.
10.Golhait P, Peseyie R. Persistent Epithelial Defect. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan
11.Diaz V. et al; ARVO 2022 Poster; A Comparison Study of the Effects of Ocular Scaffolds on Human Ocular Epithelial Cells;
12.Rutgers Benchtop Data Report: Biovance 3L Ocular; Data on file.
13.Mao Y, Protzman NM, John N, et al. An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study. J Biomed Mater Res. 2022;1‐17. doi:10.1002/jbm.b.35186.
BIOVANCE® is a registered trademark of Celularity Inc.
Verséa is a registered trademark of Verséa Health, Inc.
©2025 Verséa Ophthalmics, Inc. All rights reserved.

If you would like to learn more about Biovance 3L Ocular, please contact us at ophthalmics@versea.com
Subscribe to the Verséa Newsletter

© 2025 Verséa Health, Inc. All rights reserved.
