

Verséa Ophthalmics, Inc. is on a mission to transform clinical approaches to ocular surface diseases and ocular surgery. The company focuses on delivering biologic solutions that optimize management and treatment of various conditions, including conditions such as persistent epithelial defects, corneal dystrophies, and neurotrophic keratitis as well as surgical conditions such as pterygium excision, conjunctivochalasis, and macular hole repair.
A newly launched product portfolio consists of allograft therapeutics that will enhance clinical management that ultimately lead to better patient care.
Verséa Ophthalmics is committed to supporting Eye Care Professionals with management and treatment of patients with ocular surface diseases or requiring ocular surgery.
4 Phases of Precision Eyecare Starts with T-POC Quantitative Tear Testing
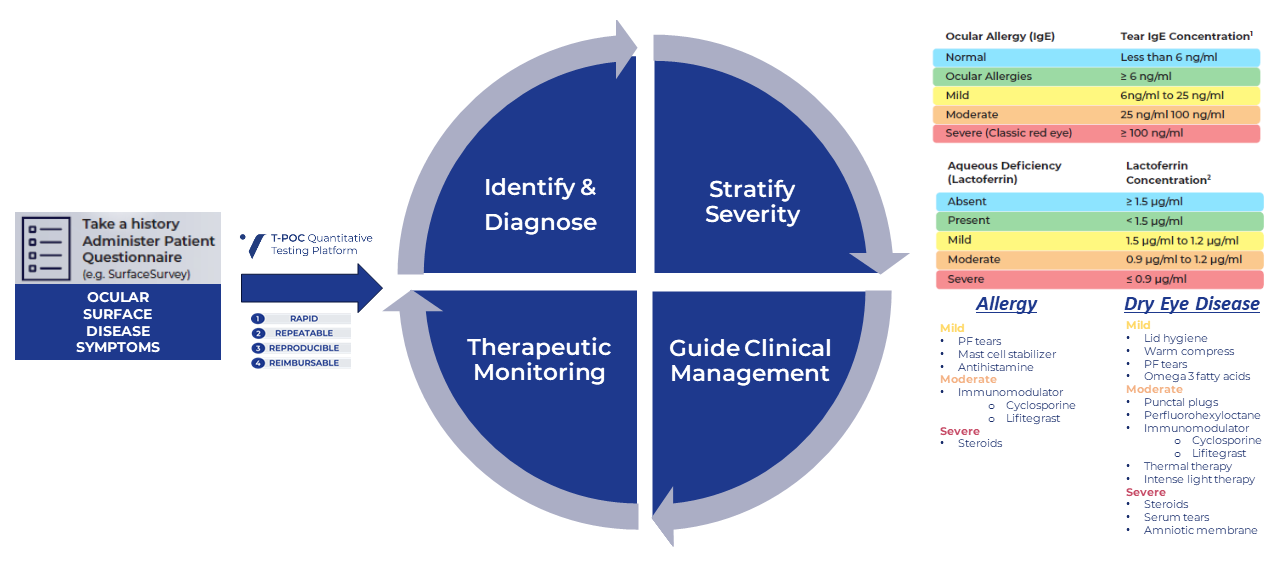
Click to Enlarge Image
Learn More About T-POC Quantitative Testing Platform
Learn More about Biovance® 3L Ocular
Verséa is a registered trademark of Verséa Health, Inc.
©2024 Verséa Ophthalmics, Inc. All rights reserved.

If you would like to learn more about Biovance 3L Ocular, please contact us at ophthalmics@versea.com
Subscribe to the Verséa Newsletter

© 2025 Verséa Health, Inc. All rights reserved.
